Acids bases and the ph scale worksheet answers – Welcome to the realm of acids, bases, and the pH scale, where the foundations of chemistry unfold. This comprehensive guide delves into the intricacies of these fundamental concepts, providing a thorough understanding through engaging explanations and detailed worksheet answers. Prepare to embark on an enlightening journey that unravels the mysteries of chemical reactions and biological processes.
As we navigate this topic, we will explore the nature of acids and bases, unravel the significance of pH in diverse fields, and uncover the practical applications of the pH scale. With each step, we will reinforce our understanding through meticulously crafted worksheet answers that illuminate the reasoning behind each solution.
1. Acids, Bases, and the pH Scale
Acids are substances that donate protons (H+ ions), while bases are substances that accept protons. The pH scale measures the acidity or alkalinity of a solution on a scale from 0 to 14. A pH of 7 is neutral, while a pH below 7 is acidic and a pH above 7 is basic.
Acids and bases play important roles in various fields, including chemistry, biology, and medicine.
Common Acids and Bases, Acids bases and the ph scale worksheet answers
- Acids: hydrochloric acid (HCl), sulfuric acid (H2SO4), nitric acid (HNO3)
- Bases: sodium hydroxide (NaOH), potassium hydroxide (KOH), calcium hydroxide (Ca(OH)2)
Importance of pH
- Chemistry: pH affects chemical reactions and the solubility of substances.
- Biology: pH is crucial for enzyme activity, cell function, and overall organism health.
- Medicine: pH is used to diagnose and treat medical conditions, such as acid reflux and kidney stones.
2. Worksheet Answers
Question 1: Define pH and explain how it is calculated.
pH is a measure of the acidity or alkalinity of a solution. It is calculated using the following formula: pH = -log[H+], where [H+] is the molar concentration of hydrogen ions in the solution.
Question 2: What is the difference between a strong acid and a weak acid?
A strong acid completely dissociates in water, releasing all of its protons. A weak acid only partially dissociates in water, releasing only a small fraction of its protons.
Question 3: Give an example of a neutralization reaction.
A neutralization reaction is a reaction between an acid and a base that results in the formation of salt and water. For example, hydrochloric acid (HCl) and sodium hydroxide (NaOH) react to form sodium chloride (NaCl) and water (H2O).
3. pH Scale Applications
Environmental Monitoring
- Measuring pH helps monitor water quality, soil acidity, and air pollution.
- pH affects the availability of nutrients for aquatic organisms and plant growth.
Medicine
- pH is essential for proper blood pH, which affects organ function and overall health.
- pH is used to diagnose and treat conditions like acidosis and alkalosis.
Agriculture
- Soil pH affects plant growth and nutrient uptake.
- Adjusting soil pH using fertilizers or lime can improve crop yields.
4. Acid-Base Reactions
Types of Acid-Base Reactions
- Neutralization: Acid + Base → Salt + Water
- Precipitation: Acid + Base → Insoluble Salt + Water
- Gas Evolution: Acid + Base → Salt + Gas
Factors Influencing Acid-Base Strength
- Ionization energy: The energy required to remove an electron from an atom.
- Electronegativity: The ability of an atom to attract electrons.
- Hydration energy: The energy released when ions are surrounded by water molecules.
Examples of Acid-Base Reactions in Everyday Life
- Baking: Baking soda (a base) reacts with acids in buttermilk or yogurt to produce carbon dioxide gas, which makes baked goods rise.
- Digestion: Stomach acid (hydrochloric acid) helps break down food.
- Cleaning: Acids (e.g., vinegar) and bases (e.g., baking soda) are used as household cleaners.
5. pH Calculations: Acids Bases And The Ph Scale Worksheet Answers
Methods of pH Calculation
- Using a pH meter
- Using a pH indicator
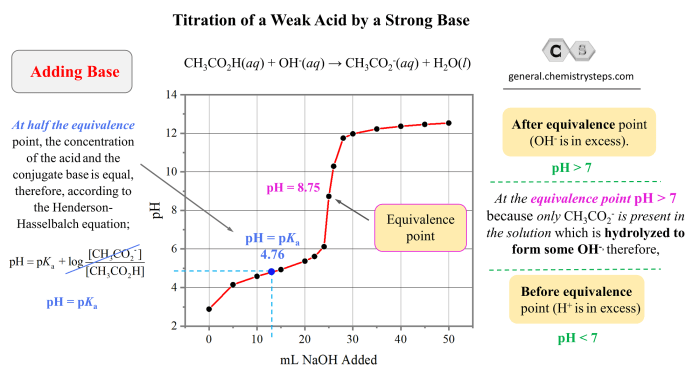
- Using the Henderson-Hasselbalch equation: pH = pKa + log([A-]/[HA]), where pKa is the acid dissociation constant, [A-] is the concentration of the conjugate base, and [HA] is the concentration of the acid.
Limitations of pH Calculations
- pH calculations assume ideal conditions and may not be accurate in non-ideal solutions.
- pH calculations do not consider the effects of temperature and ionic strength.
Examples of pH Calculations in Real-World Scenarios
- Calculating the pH of blood to diagnose acidosis or alkalosis.
- Calculating the pH of soil to determine the need for lime or fertilizer.
- Calculating the pH of wastewater to ensure compliance with environmental regulations.
Helpful Answers
What is the pH scale?
The pH scale is a logarithmic scale used to measure the acidity or alkalinity of a solution. It ranges from 0 to 14, with 0 being the most acidic and 14 being the most alkaline (basic).
Why is pH important?
pH plays a crucial role in various fields, including chemistry, biology, and environmental science. It affects chemical reactions, enzyme activity, and the survival of organisms.
How do I calculate pH?
pH can be calculated using different methods, including using a pH meter, litmus paper, or mathematical equations based on the concentration of hydrogen ions.