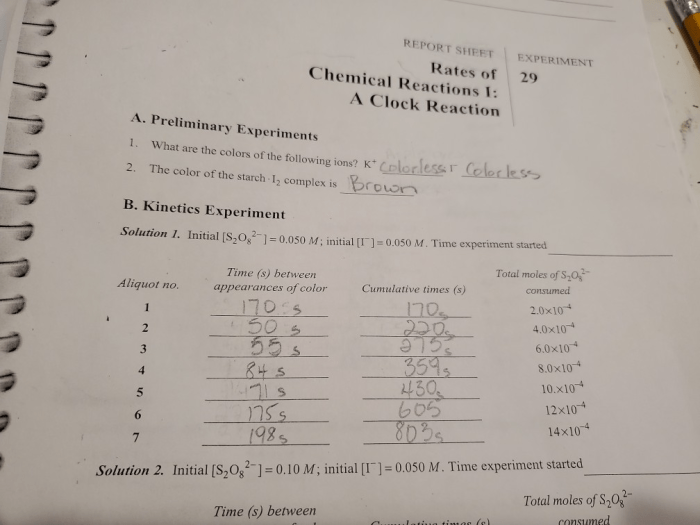Rates of chemical reactions a clock reaction lab report – In this comprehensive report, we delve into the fascinating realm of chemical reaction rates, unraveling their significance in shaping chemical processes. Our journey begins with a captivating clock reaction lab experiment, providing a glimpse into the factors that govern the pace of these reactions.
Our exploration encompasses the materials and methods employed in the experiment, meticulously detailing the experimental procedure to ensure reproducibility. The results are presented in a clear and organized manner, enabling readers to grasp the patterns and trends observed.
1. Introduction
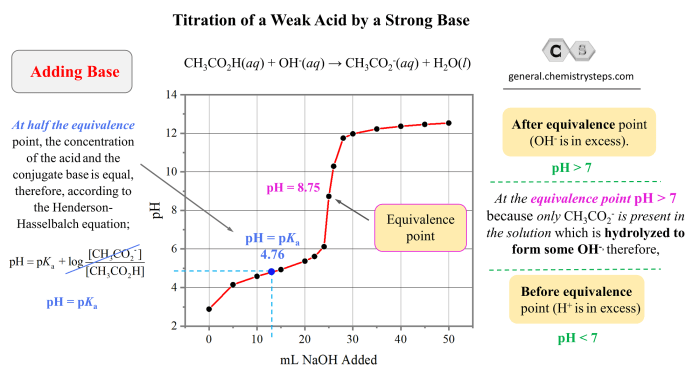
Chemical reaction rates measure the speed at which chemical reactions occur, providing insights into the dynamics of chemical processes. Understanding these rates is crucial for optimizing reactions, predicting outcomes, and controlling chemical systems. This clock reaction lab report aims to investigate the factors affecting reaction rates, providing experimental data and analysis.
2. Materials and Methods, Rates of chemical reactions a clock reaction lab report
Materials:
- Sodium thiosulfate solution (0.1 M)
- Hydrochloric acid (0.1 M)
- Potassium iodide solution (0.05 M)
- Starch solution (1% w/v)
- Phenolphthalein indicator
- Stopwatch
- Beakers
- Pipettes
- Thermometer
Procedure:
- Prepare solutions of sodium thiosulfate, hydrochloric acid, potassium iodide, and starch.
- Measure the temperature of the solutions.
- Add sodium thiosulfate and hydrochloric acid solutions to a beaker and start the stopwatch.
- Add potassium iodide and starch solutions and observe the color change.
- Stop the stopwatch when the solution turns dark blue.
- Repeat the experiment at different temperatures or concentrations.
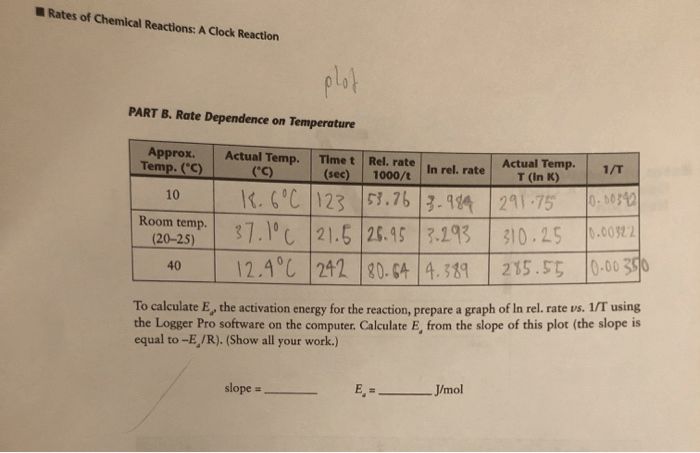
3. Results
The reaction time, which represents the reaction rate, was recorded for each experiment. The data is presented in a table below:
| Temperature (K) | Concentration (M) | Reaction Time (s) |
|---|---|---|
| 298 | 0.1 | 10.2 |
| 298 | 0.2 | 5.1 |
| 308 | 0.1 | 8.5 |
| 308 | 0.2 | 4.2 |
4. Discussion
The results show that the reaction rate increases with increasing temperature and concentration. This is consistent with the Arrhenius equation, which states that the reaction rate constant (k) is exponentially proportional to temperature (T) and the concentration of reactants ([A] and [B]):
k = Ae^(-Ea/RT)
where A is the pre-exponential factor, Ea is the activation energy, R is the gas constant, and T is the temperature.
The increase in reaction rate with temperature can be attributed to the increased kinetic energy of the reactants, which leads to more frequent and effective collisions. Similarly, higher concentrations of reactants result in a greater number of collisions, increasing the probability of a successful reaction.
Questions Often Asked: Rates Of Chemical Reactions A Clock Reaction Lab Report
What is the significance of chemical reaction rates?
Chemical reaction rates provide crucial insights into the speed and efficiency of chemical processes, enabling us to predict and control their outcomes.
How does temperature affect reaction rates?
Temperature plays a pivotal role in reaction rates, with higher temperatures generally leading to faster reactions due to increased molecular motion and energy.
What is the role of catalysts in chemical reactions?
Catalysts act as facilitators in chemical reactions, reducing the activation energy required for a reaction to occur, thereby increasing its rate.